Projects
We study how pancreatic islet cell-to-cell interactions shape oscillatory Ca$^{2+}$ activity, balancing hormone secretion and maintaining blood glucose homeostasis by employing microfluidic experiments and mathematical modeling.
Background
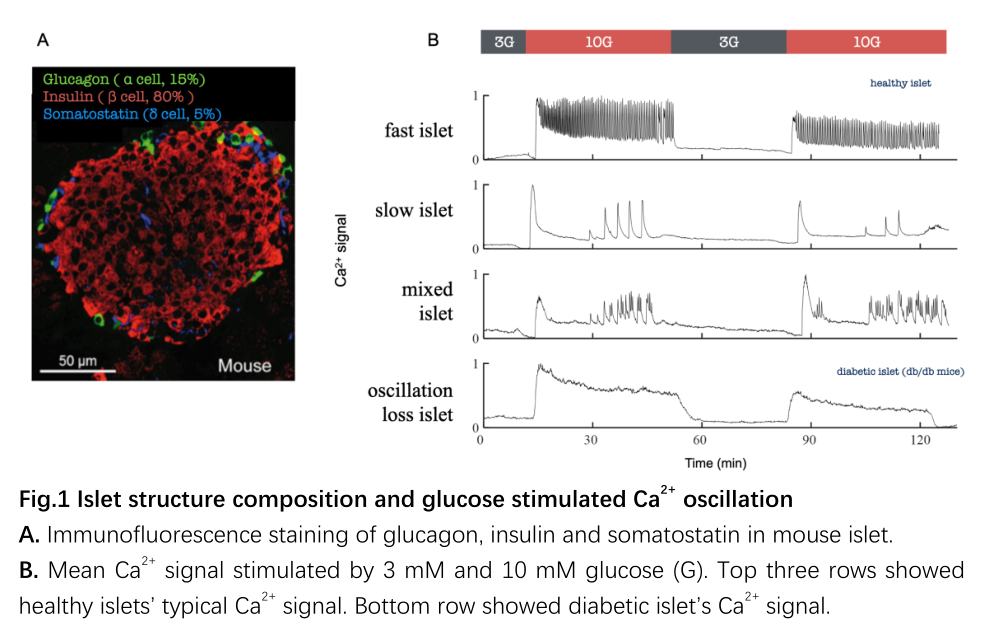
The pancreatic islets are the most crucial blood glucose regulatory micro-organ in the body, primarily distributed in the head and tail of the pancreas (approximately 1,000 islets per mouse and about 1,000,000 islets per human). Each islet exhibits an independent hierarchical structure (Fig. 1A): approximately 5% of δ-cells (secreting somatostatin) and about 15% of α-cells (secreting glucagon) are mainly distributed on the surface, while around 80% of β-cells (secreting insulin) are concentrated in the center, with abundant paracrine regulation. Functionally, following high glucose stimulation, the islets display regular Ca2+ oscillations, categorized into fast, slow, and mixed oscillation patterns (Fig. 1B). The stability of islet Ca2+oscillation is highly correlated with the progression of diabetes; thus the regulatory mechanisms have been widely studied.
Previous physiological research has primarily focused on the most abundant β-cells, while the less numerous α-cells and δ-cells have long been neglected. Moreover, most studies separate the secretion of glucagon by α-cells (raising blood sugar) from the secretion of insulin by β-cells (lowering blood sugar), such as activating β-cells under high glucose and α-cells under low glucose. However, within the body, these two types of cells are spatially intermingled and functionally interact each other through feedback mechanisms. This suggests that both hormones may be dynamically modulated by three types of islet cells simultaneously. Additionally, hypoglycemia is frequent in late-stage type 1 and type 2 diabetes, indicating significant abnormalities not only in insulin but also in glucagon function in patients. Therefore, the onset of the disease may stem from abnormalities in the regulation of both hormones.
Featured
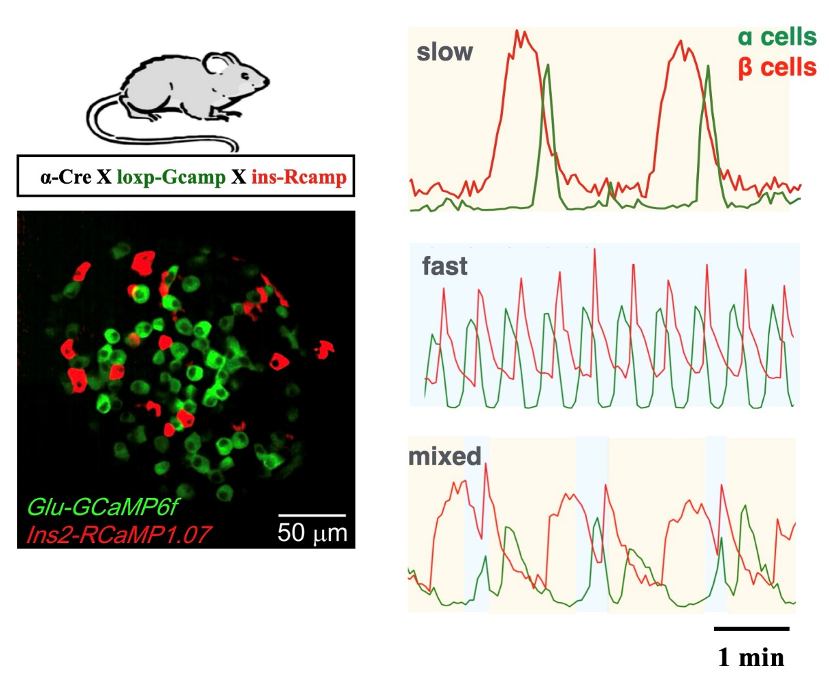
By developing a microfluidic islet experimental system and generating transgenic mice with dual-color Ca2+ markers for α and β cells, it was observed islet α and β cells exhibited global phase locking of Ca2+ activity.

Using a novel extracellular Zn2+ probe to observe insulin vesicle secretion, it was found that the secretion capacity of islet β cells exhibits heterogeneity. Pharmacological blockade of δ cells disrupted the exponential distribution, leading to a more uniform secretion capacity among cells.
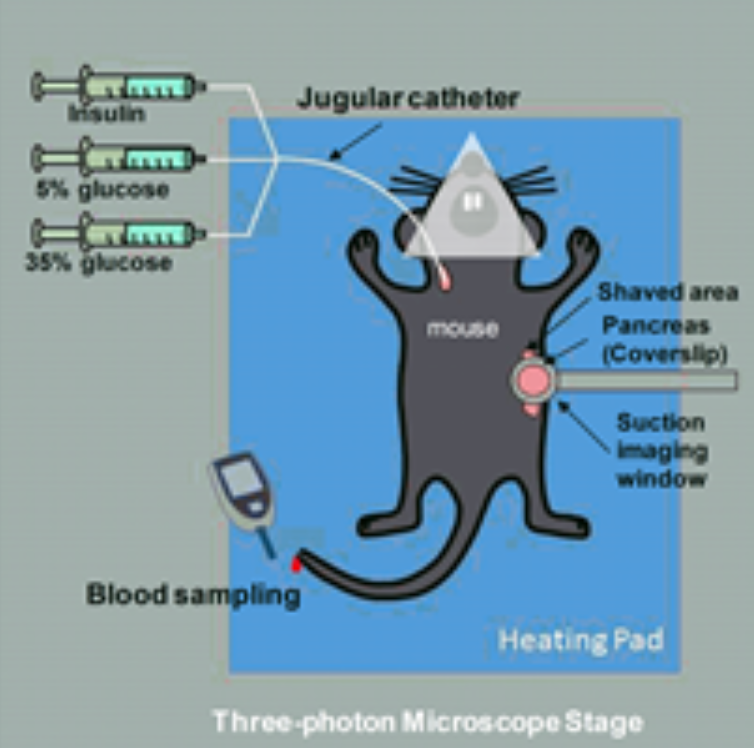
We developed in vivo pancreatic islet Ca2+ functional imaging using two-photon microscopy. Together with in situ pancreas islet imaging, we also developed kidney capsule transplantation imaging system.
Despite constituting only 5% of islet cells, delta cells’ functions have been underestimated in previous studies. Continuous adjustment of δ-α coupling strength caused the fast oscillating islets to transition to mixed and slow oscillations.
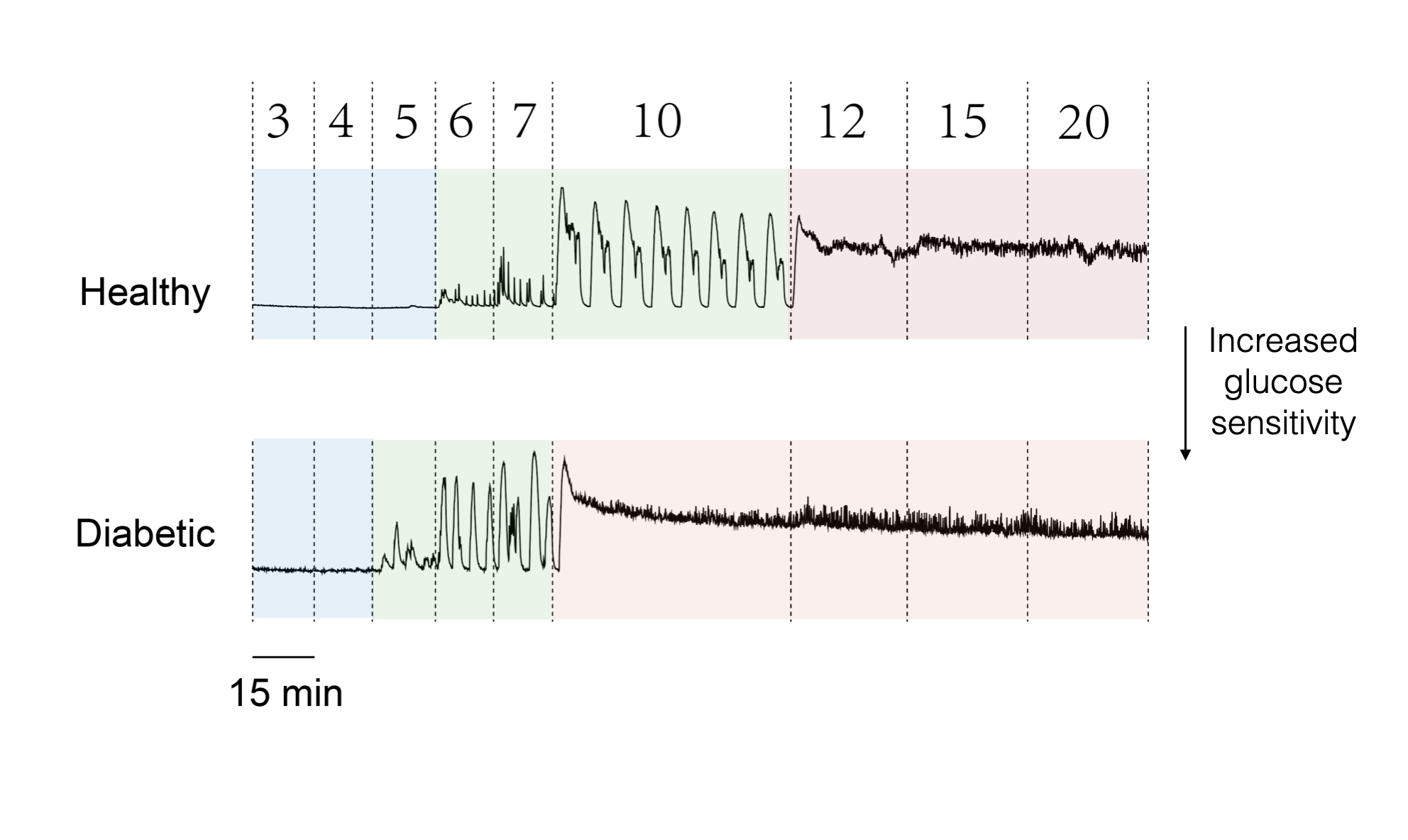
Pancreatic islets from healthy mice exhibit regular Ca2+ oscillations, while those of diabetic mice show a cessation of Ca2+ oscillation activity. We wish to explore the molecular mechanisms that impairs the oscillation in diabetic islets.